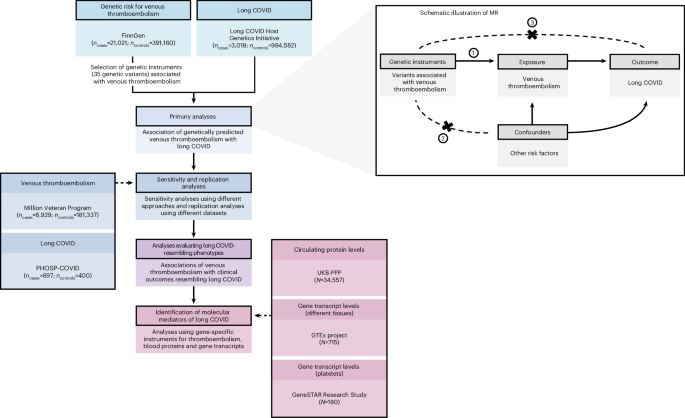
Long COVID, characterized by persistent symptoms after acute COVID-19 infection, poses a significant health burden worldwide. Recent genetic research reveals that thromboembolism—a condition involving blood clots—may play a crucial role in the pathogenesis of long COVID in individuals of European ancestry.
A comprehensive genome-wide association study (GWAS) conducted using data from the FinnGen biobank identified significant genetic loci linking long COVID with venous thromboembolism (VTE). Researchers applied Mendelian randomization techniques to infer causality, demonstrating that genetic predisposition to thromboembolism increases the risk of developing long COVID symptoms. This supports previous clinical observations of coagulopathy and persistent clotting abnormalities in post-COVID conditions (Lammi et al., Nat Genet).
Genetic Evidence Linking Thromboembolism and Long COVID
- GWAS analysis pinpointed variants in genes associated with coagulation pathways, including those affecting platelet function and thrombin receptor signaling.
- Colocalization analyses confirmed that these genetic signals overlap between thromboembolic disorders and long COVID susceptibility, indicating shared biological mechanisms.
- Functional annotation suggests involvement of protease-activated receptor 1 (PAR-1), critical for thrombin-mediated platelet activation and endothelial response.
Thromboinflammation, the interplay between thrombosis and inflammation, emerges as a key pathological process. Studies reveal persistent endothelial dysfunction and microclots in long COVID patients, which sustain inflammation and may contribute to neurological and respiratory sequelae (Cervia-Hasler et al., Science). Importantly, genetic findings corroborate these mechanisms by showing inherited factors that modulate thrombotic response influence long COVID risk.
Clinical and Therapeutic Implications
Understanding thromboembolism’s role opens new avenues for prevention and treatment. Anticoagulant therapies could potentially reduce long COVID incidence in genetically susceptible individuals, although clinical trials are needed. The integration of human genetics with proteomics and clinical data may enable targeted interventions and personalized medicine approaches (Henry et al., Circulation).
This research highlights the value of large-scale biobank studies and sophisticated genetic methodologies, such as Mendelian randomization, to unravel complex disease etiologies. By leveraging genetic evidence, scientists can identify actionable pathways relevant to long COVID and improve patient outcomes.
Future studies should expand analyses across diverse ancestries and explore gene-environment interactions to fully elucidate the thromboembolic contribution to long COVID globally (Vos et al., JAMA). These insights are critical for informing public health strategies and developing effective therapies for the growing population affected by long-term COVID-19 complications.
Read more at: www.nature.com